Heavy-liquid-metal-based heat transfer fluids for high-temperature solar and nuclear power applications
Liquid metals have been studied since the early development of fission energy as reactor core coolants for fast reactors, fusion energy blanket applications and, more recently, for both accelerator-driven systems (ADS) proposed for high-level radioactive waste transmutation and for generation IV fast reactors. Moreover, heavy liquid metals (HLM) are being proposed as target materials for high-power neutron spallation sources. In last two decades, HLM were studied also as a potential candidates for heat transfer fluids in concentrating solar power (CSP) systems.
The general selection criteria for the use of liquid metals as heat-transfer fluids include the following [1]:
-acceptable corrosion and mechanical degradation of structural materials (i.e. lifetime of equipment)
-high stability of liquid metal (limited chemical reactions with secondary coolants and air or formation of spallation products);
-moderate power requirement for liquid metal circulation ;
-high heat transfer coefficient and small size of heat exchanger;
-controllable chemical and radioactive hazards;
-simple and reliable safety measures and systems.
Besides that, in a nuclear environment, there are several more requirements related to the fast spectrum necessary for breeding, fuel conversion, and actinide transmutation [1]:
-small (fast) capture cross-section;
-high scattering cross-section (for small leakage of neutrons from the core);
-small energy loss per collision (for small spectrum softening [moderating] effect);
-high boiling temperature (for prevention of reactivity effects from boiling-related coolant voiding)
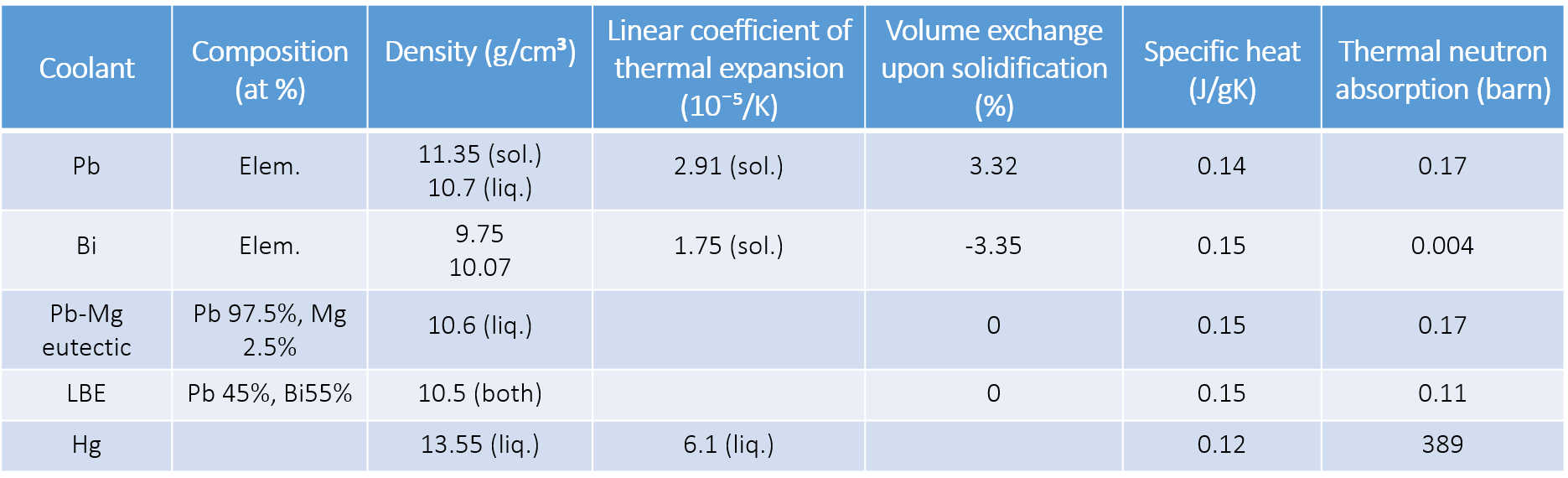
Some relevant properties of heavy liquid metal candidate materials [1, 2]
[1] Handbook on lead-bismuth eutectic alloy and lead properties, Materials Compatibility, Thermal Hydraulics and Technologies, OECD – NEA 7268, ed. 2015.
[2] D. Frazer, E. Stergar, C. Cionea, P. Hosemann, Liquid metal as a heat transport fluid for thermal solar power applications, Energy Procedia 49 (2014) 627–636.
Many engineering environments are non-ambient. In order to understand how a material reacts in a non-ambient environment, simulated and well-controlled conditions must be applied. Our research team focuses on high-temperature and liquid-metal environments, such as can be found in liquid-metal-cooled reactors and solar concentrators. The autoclaves are used to carry out long-term testing to evaluate corrosion rates. The material degradation is subsequently characterized through microscopy studies.

Current efforts are focusing on high-temperature heavy metals (e.g., lead-bismuth eutectic) in static, oxygen-controlled environments. The aim of this project is to provide sufficient oxygen in the liquid metal such that the submerged steel can form a protective oxide layer that prevents catastrophic dissolution.
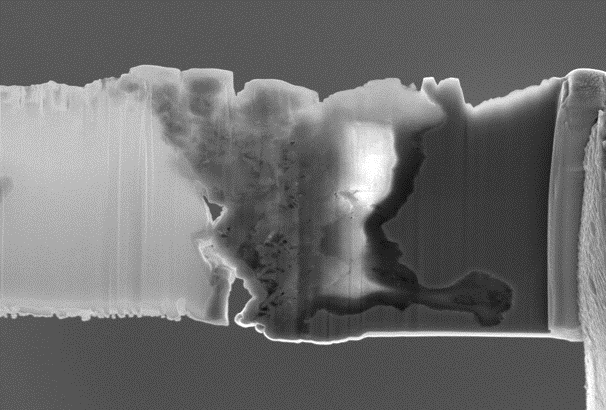
In addition to liquid-metal corrosion, oxidation of reactor-grade materials in high-temperature steam environments is of interest to aid the development of accident-tolerant fuels. Fe-Cr-Al alloys as well as Mo and SiC are investigated for this purpose.
